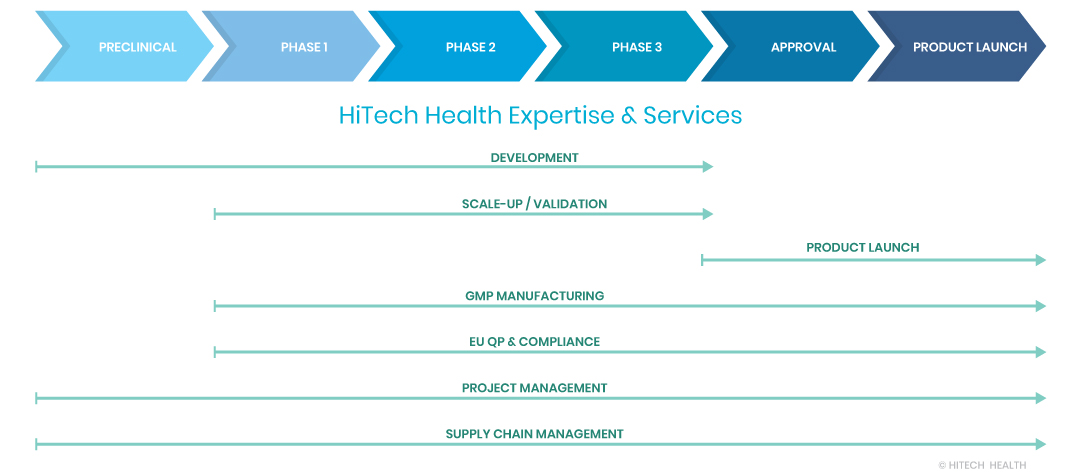
Contract Development and Manufacturing
Organisation (CDMO)
Qualified Person (QP), Quality & Supply
Chain Services
We can provide batch importation and Qualified Person (QP) release services for both the EU and UK markets.
Our Clients
Enabling healthcare companies to launch and supply products
Cell & Gene Therapy | Pharmaceutical | Biotechnology | Medical Device
Your Commercialisation Journey