The Advanced Therapeutic Medicinal Products (ATMPs) sector is evolving rapidly and new products have already demonstrated the ability to reverse or significantly impact the progression of disease. In recent years, the advent of cell and gene therapies has shown the possibility of providing transformative and potentially curative outcomes for a diverse range of diseases and injuries.
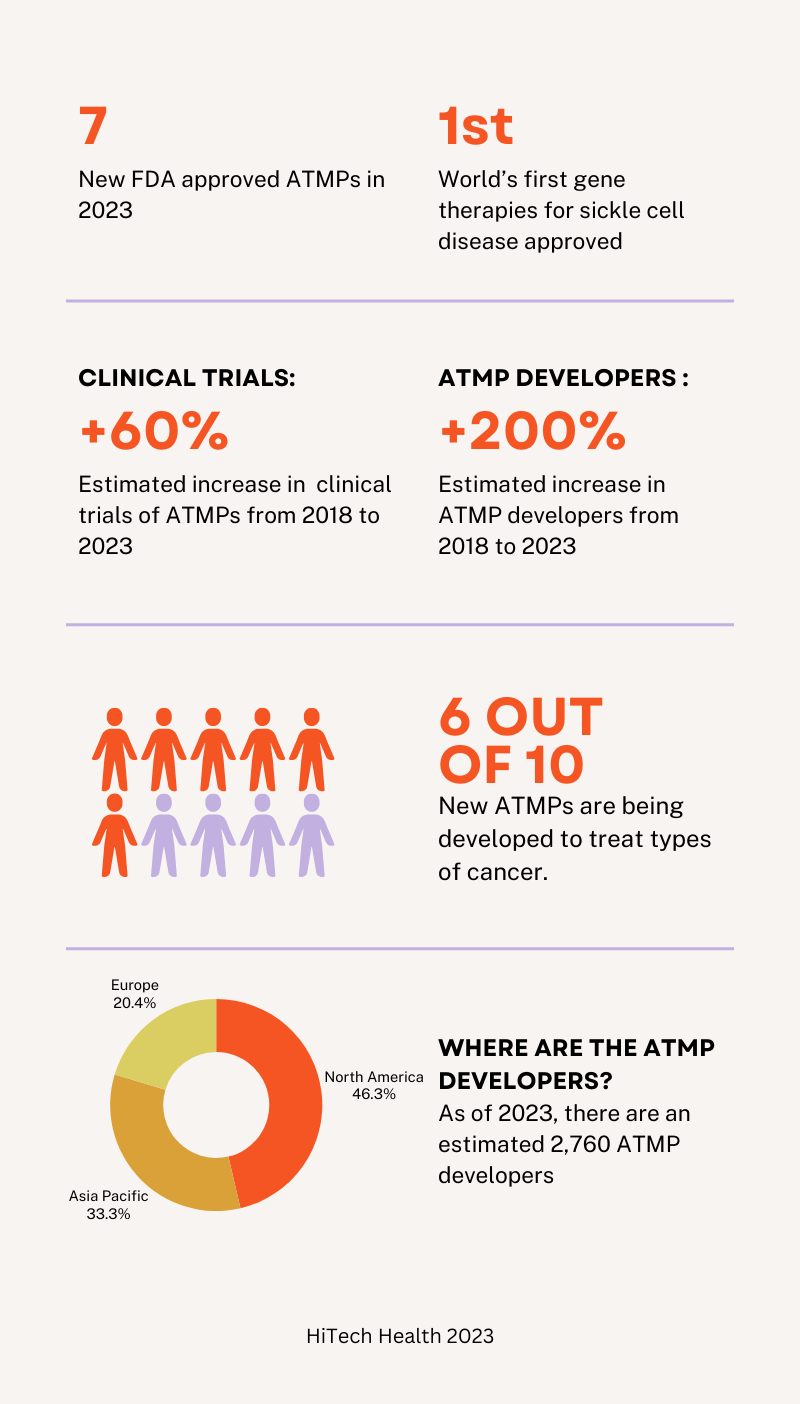
At the end of 2022, there were 26 cell and gene therapies approved in the United States by the FDA. In 2023, an additional 7 cell and gene therapies were approved bringing the total number to 33 approved therapies (1). The largest sub-class of approved therapies are umbilical cord blood derivatives, representing 8 of the 32 cell and gene therapy approvals to date. CAR-T cell therapies represent the next largest segment, composing 6 of the 32 cell and gene therapy approvals (2). The approved CAR-T cell therapies in the U.S. include: Abcema, Breyanzi, Carvykti, Kymriah, Tecartus, and Yescarta.
In December 2023, the FDA approved the first gene therapies, Casgevy and Lyfgenia, for sickle cell disease in patients 12 years or older. Casgevy was developed through a partnership with Vertex Pharmaceuticals and CRISPR Therapeutics while Lyfgenia was developed by Bluebird Bio. Both therapies are made from the patients’ own blood stem cells, which are modified, and are given back as a one-time, single-dose infusion as part of a hematopoietic (blood) stem cell transplant. Prior to treatment, a patients’ own stem cells are collected, and then the patient must undergo myeloablative conditioning (high-dose chemotherapy), a process that removes cells from the bone marrow so they can be replaced with the modified cells in Casgevy and Lyfgenia (3). Casgevy was already approved by the UK’s MHRA on 15th November 2023.
The 33 cell and gene therapies that have been approved by the FDA represent approximately 9% of the estimated 359 approved biologics (4). Although this is a small percentage it does mark significant growth considering the first FDA approval of a cell and gene therapy occurred in 2017, Kymriah.
The FDA has identified a requirement for additional qualified people to manage the growing number of cell and gene therapy submissions. There are currently more than 1,000 cell and gene therapies in clinical development in the U.S., with more than 3,000 in pre-clinical development (5). To meet the growing demands, the US congress authorised new funding for the agency when it reauthorised the new Prescription Drug User Fee Act (PUFA VII) in 2022. This has allowed the FDA to pursue the hiring of new employees for its newly created ‘super office’, named the Office of Therapeutic Products, which replaced the Office of Tissues and Advanced Therapies.
New offices created within the super office structure align disciplines and product types, aiming to allow the FDA’s workforce to address the exponential growth in cell and gene therapies. The transition to the new Office of Therapeutic Products aims to help the FDA meet its expectation of approving 10-20 cell and gene therapies annually by 2025.
In Europe, the EMA had approved 24 ATMPs by the end of 2022, although 7 of the products were subsequently withdrawn from the market or else did not have their Marketing Authorisation (MA) renewed (6). The first ATMP to be approved by the EMA in 2023 was Hemgenix. Hemgenix was developed by Dutch biotechnology company UniQure to treat adults with severe and moderately severe haemophilia B, an inherited bleeding disorder caused by the lack of factor IX (a protein needed to produce blood clots to stop bleeding) (7).
In January 2022, Regulation (EU) 2021/2282 on health technology assessment (HTA) came into effect. The regulation aims to be applied by 12th January 2025 and will therefore take three years to become effective in Member States. The HTA introduces an evidence based process that will allow the competent EU and national authorities to determine the effectiveness of new or existing health technologies, including cell and gene therapies. From 2025, all cell and gene therapies will undergo a single EU assessment of the value they add to patients and healthcare systems, aiming to end the need for 27 individual reviews. Companies will also meet jointly with the European Medicines Agency and Europe’s HTA coordinating group to discuss and align on the optimal clinical trial designs that deliver data, not only on safety and efficacy but also on added value to the patients and healthcare systems (8, 9).
In 2018, there were approximately 1000 clinical trials and 900 developers according to the Alliance for Regenerative Medicine. As of 2023, there are an estimated 1,687 clinical trials that are on-going and an estimated 2,760 developers. Of the 2760 developers, 1,235 are based in North America, 888 are based in Asia Pacific and 543 are based in Europe. Of the current active clinical trials, 917 trials are in North America, 648 are in the Asia Pacific region and 329 are in Europe (10).
The majority of the clinical trials involve cell therapies or gene-modified cell therapies, accounting for approximately two thirds of the trials. The rest of the trials involve DNA and RNA therapeutics, gene therapies and genone editing. Types of cancer are the most common therapeutic indication, approximately 60% (10).
The data from 2023 shows clear growth in the ATMP sector that is yielding positive outcomes for patients. Over the last several years, the ATMP sector has seen consistently increasing numbers of clinical trials, increases in investment, partnerships, and year-on-year growth in regulatory approvals by both the FDA and the EMA. Challenges undoubtedly lie ahead in 2024 but it is clear that ATMPs are helping patients with unmet medical needs. The strong pipeline of future therapies requires continuous innovation and investment by the regulatory agencies, developers and manufacturers to meet the industry’s requirements.
Hitech Health is a leading European CDMO and service provider for ATMPs including cell and gene therapies. If you require support with the development and manufacturing of an ATMP, schedule a meeting with our team by emailing info@hitech-health.com.
Author: Paul Crozier
Date: 13th December 2023
References
- fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
- bioinformant.com/u-s-fda-approved-cell-and-gene-therapies
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
- cellandgene.com/doc/s-market-outlook-for-cell-and-gene-therapies-0001
- biospace.com/article/fda-braces-for-looming-boom-in-cell-and-gene-therapy-submissions
- ema.europa.eu/en/documents/report/cat-quarterly-highlights-approved-atmps-may-2023_en.pdf
- ema.europa.eu/en/medicines/human/EPAR/hemgenix
- https://www.europeanpharmaceuticalreview.com/article/173762/the-eu-hta-regulation-a-new-frontier-for-access-to-innovative-technologies/
- politico.eu/article/europe-afford-next-generation-medicine/#:~:text=me%20the%20money-,To%20date%2C%20the%20European%20Commission%20has%20authorized%2025%20cell%20and,and%20certain%20types%20of%20cancer.
- alliancerm.org/the-sector-snapshot-august-2023/

