by Paul Crozier | Jan 22, | HiTech Health Latest News
By 2025, the global advanced therapy medicinal products (ATMP) sector, encompassing cell therapies, gene therapies, and genetically modified cell-based medicines, has progressed from early scientific success to clinical and commercial reality. Following a decade of rapid innovation, the ATMP sector is now characterised by a growing number of approved therapies, expanding therapeutic indications, and increasing global competition. The patient benefits of ATMPs are undeniable with a year-on-year growth in the number of patients who are benefitting from advanced therapies globally. Patients who previously had no treatment options are now being offered renewed hope through new innovative ATMPs. At the same time, the sector has faced commercial hurdles in 2025 including manufacturing and supply chain challenges, pricing and reimbursement scrutiny, and a renewed emphasis on demonstrating sustainable clinical and economic value at scale.
In 2025, 20 of the 30 largest biopharma companies by market cap are now investing in the development and commercialisation of ATMPs. Research conducted by Citeline reported ATMP sector funding of $11.1 Billion in 2025. The ATMP sector comprised approximately 18% of the value of all biotech venture financing in 2025, an increase in the reported 15% value report in 2024.
The Race to the Clinic: Asia Pacific Region Surpasses North America
In the past year, the Asia-Pacific region overtook North America for the first time in the number of ATMP clinical trials, signalling a significant shift in the global advanced therapy landscape. There were an estimated 916 clinical trials ongoing in North America in 2025, with 890 of these in the United States, while the Asia-Pacific region conducted approximately 990 trials, 716 of them in China. This growth in the Asia-Pacific region reflects the region’s growing focus on accelerating the translation of novel therapies from lab to clinic, supported by evolving government policies that are driving faster initiation of in-human studies. The trend highlights a broader intensifying competition to bring breakthrough ATMPs to patients worldwide.
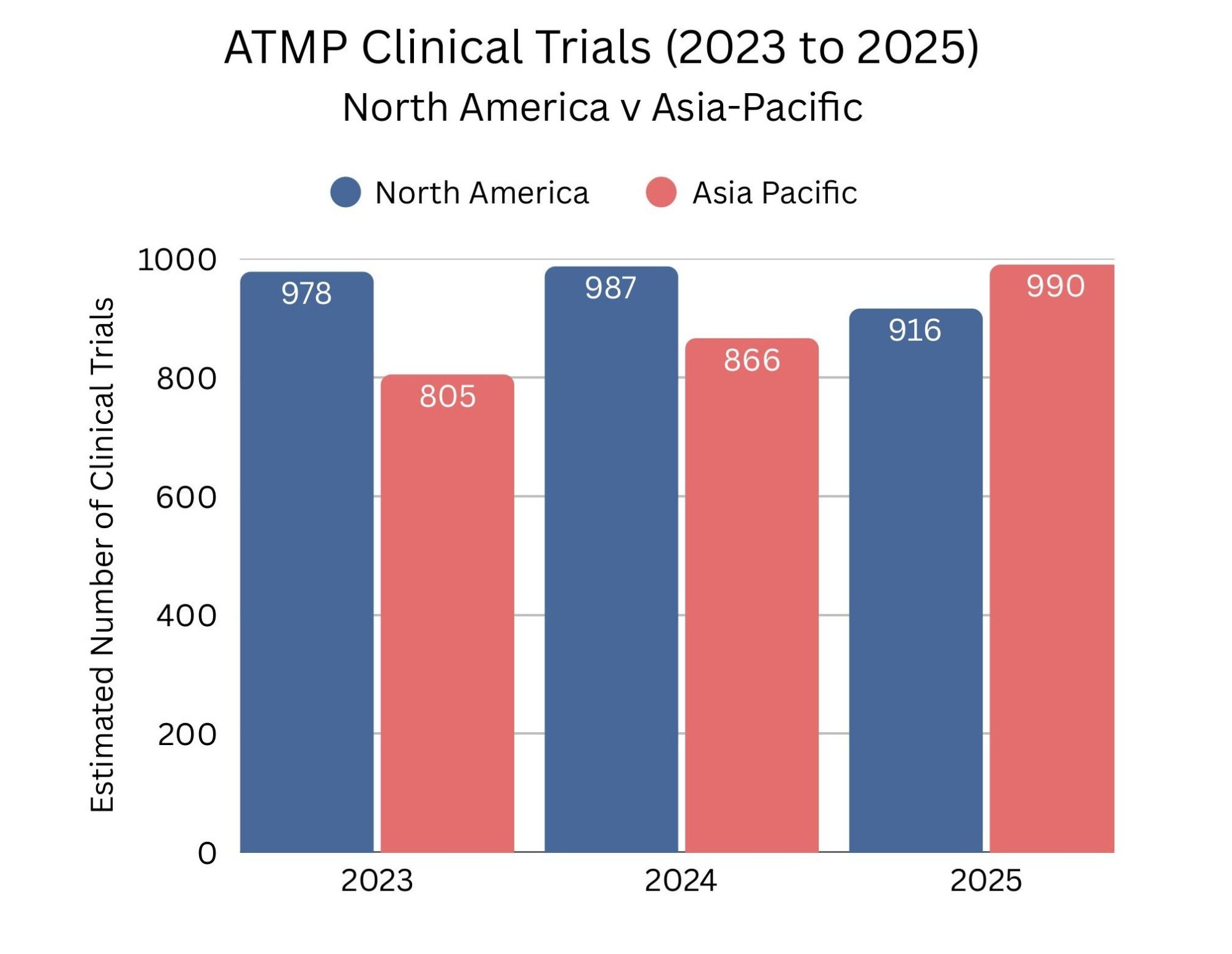
Source data: www.alliancerm.org
FDA and EMA Approvals in 2025
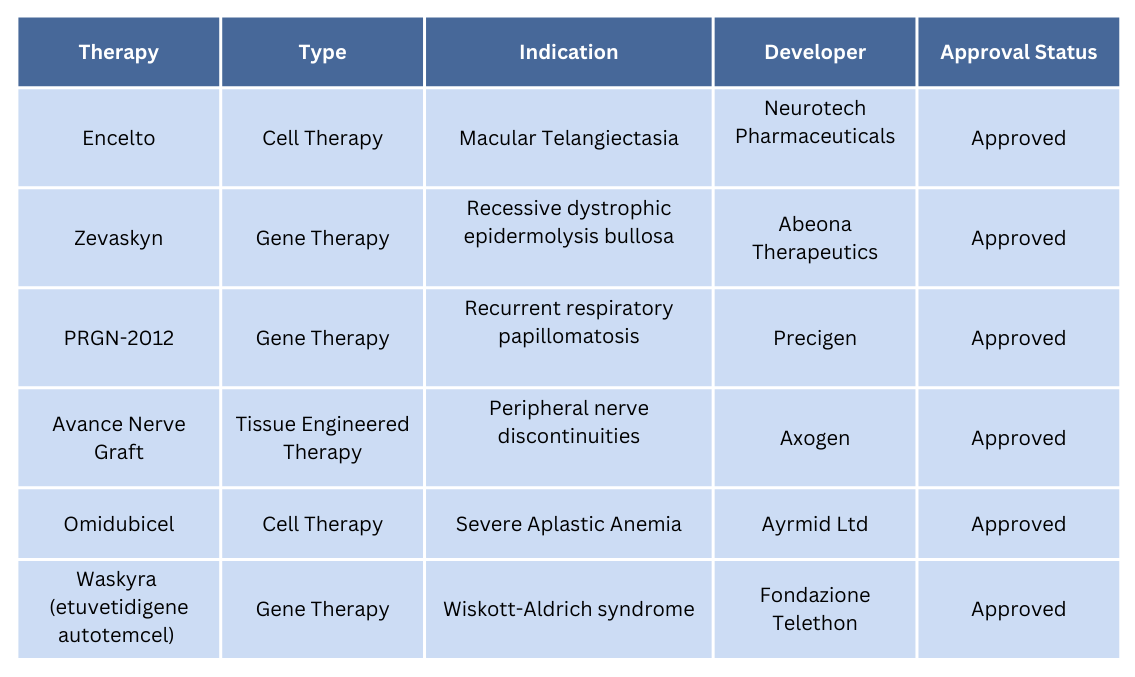
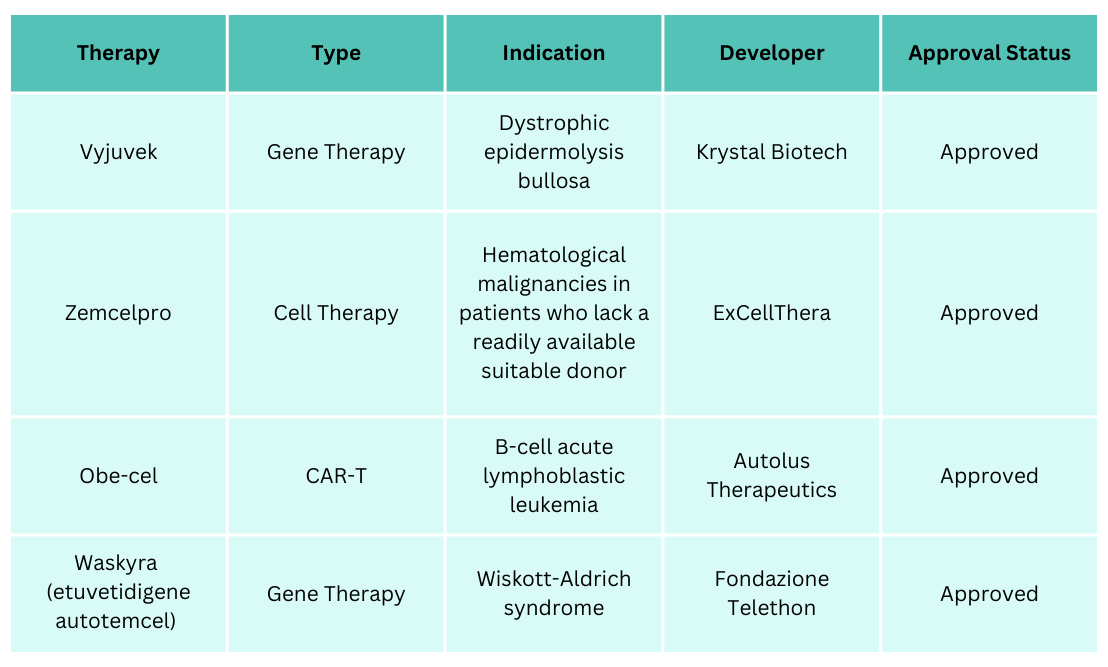
Over the past year, both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) granted several significant approvals for ATMPs. In total, the FDA approved six ATMPs, while the EMA approved four. A major milestone in 2025 was the approval of Fondazione Telethon’s etuvetidigene autotemcel (Waskyra) for Wiskott–Aldrich syndrome (WAS) by both the FDA and EMA. Waskyra is the first gene therapy approved for this rare disease, and Fondazione Telethon became the first non-profit organisation to achieve gene therapy approval. The tables below provide details on these newly authorised ATMPs.
ATMPs that were approved by the FDA in 2025

ATMPs that were approved by the EMA in 2025

Mesoblast’s Ryoncil became commercially available in the US in March 2025
At the end of 2024, Melbourne-based biotech Mesoblast secured FDA approval for Ryoncil, the first mesenchymal stromal cell (MSC) therapy to be approved by the FDA for any indication. Ryoncil is now an approved treatment for children aged two months and older, including adolescents, with steroid-refractory acute graft-versus-host disease (SRaGvHD), a life-threatening condition associated with high mortality. The therapy became commercially available in the US on 28th March 2025. Sales of Ryoncil reached US$13.2 million in the second quarter of 2025, increased to US$21.9 million in the third quarter (a 66% quarter-on-quarter increase), and rose further to US$35.1 million in the fourth quarter (approximately 60% growth over the previous quarter). This accelerating commercial performance reflects the growing number of patients benefiting from this MSC-based therapy.
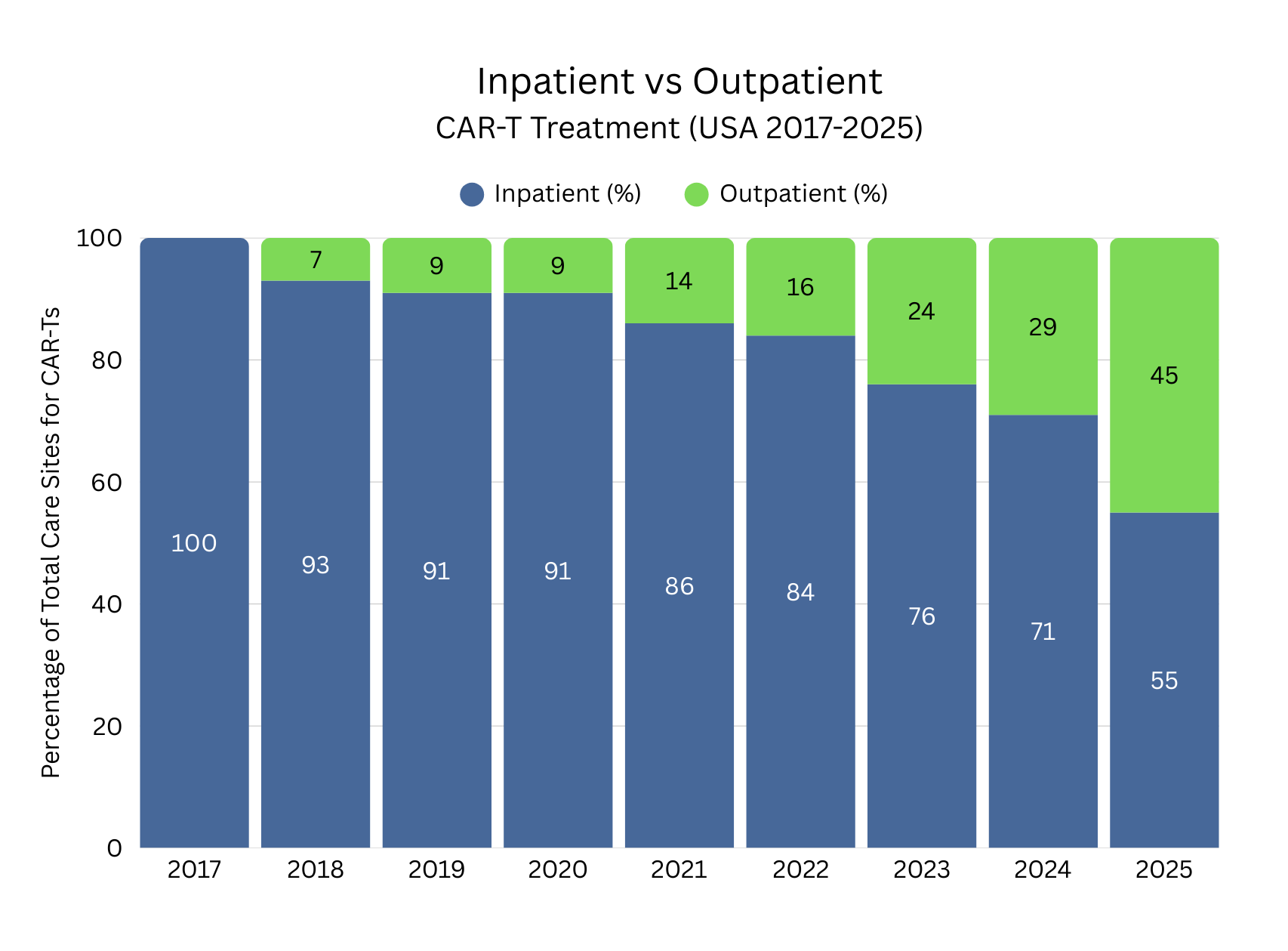
Significant Growth in Outpatient CAR-T Treatment
Among the positive developments has been the continued expansion of ATMP treatment access in outpatient settings. Outpatient CAR-T treatment has experienced significant growth in recent years, reflecting increasing understanding and clinical education on CAR-T administration, increased safety profiles, improved toxicity management and adapting healthcare infrastructure. Continuous improvements by developers and healthcare teams have made it possible to safely transition appropriate patients from inpatient to outpatient settings. Standardised monitoring protocols, remote symptom tracking, and rapid-response care pathways have increased clinician confidence in outpatient delivery while reducing hospital length of stay and overall treatment costs. This trend has enhanced patient convenience and quality of life, eased capacity constraints in hospitals, and positioned outpatient CAR-T therapy as an increasingly viable treatment option for some patients.

Source data: Guidehouse Analysis of McKesson Compile Patient Ready Data (US)
European Commission Proposes Biotech Act to Strengthen EU Health Biotechnology
The European Commission published its proposal for a European Biotech Act in December 2025, as outlined in its 2024–2029 Political Guidelines. The Act seeks to create a supportive framework to accelerate the development of biotechnology products from research to market, while maintaining high safety and ethical standards. It represents a potential important milestone for ATMPs in Europe by seeking to better align regulation, investment, and patient access.
The Act combines regulatory improvements, targeted incentives, and funding measures to boost clinical trial activity, support ATMP centres of excellence, and enhance intellectual property protection, including a 12-month extension of supplementary protection certificates. It also aims to address Europe’s long-standing challenges in translating scientific excellence into commercial success, particularly in health biotechnology, where complex legislation and funding gaps had led to many developers focusing on other markets such as North America
Here are three main takeaways from the recently adopted Act:
- The Biotech Act seeks to address structural barriers limiting Europe’s ability to commercialise biotech innovation, including funding constraints and a declining share of global clinical trials.
- It introduces coordinated measures across seven core pillars, including strategic project designations, fast-track permitting, additional funding, and extended SPCs for qualifying biotech and ATMP products.
- The Act streamlines existing EU regulatory frameworks (including CTR, ATMP, and SoHO) through accelerated timelines, risk-based requirements, and regulatory sandboxes, aiming to reduce time to market while preserving high safety standards.
In parallel with the Biotech Act proposal, the Commission and the European Investment Bank Group announced BioTechEU, an initiative to mobilise €10 billion in public-private investment for the biotech and life sciences sectors in the next year.
ATMP Companies are Adapting Fast
To address the challenges within the ATMP sector, companies are increasingly required to adapt quickly and operate with greater agility in a rapidly evolving landscape. A growing number of developers are focusing on delivering best-in-class advanced therapies for indications with high unmet need and significant patient populations, including Parkinson’s disease, Multiple myeloma, Huntington’s disease, and others. This shift reflects both scientific progress and a broader commitment to addressing diseases where conventional treatment options remain limited.
In the early days of the ATMP sector, access to treatment was constrained by significant operational and infrastructure barriers, including a limited number of specialised treatment centres, Today, the sector is actively working to dismantle these barriers and expand patient access. For example, Carvykti, a personalised CAR-T cell therapy for adults with relapsed or refractory multiple myeloma, is now administered in an outpatient setting approximately 50% of the time. Developers are collaborating closely with regulatory agencies to enhance safety profiles and refine clinical trial designs in response to regulatory feedback, supporting more efficient and patient-centric development pathways. Heading into 2026, a significant number of regulatory decisions and submissions are planned for first half of the year and global clinical trials are gaining momentum.
HiTech Health is a leading European CDMO and service provider for ATMPs. We deliver end-to-end solutions from preclinical development through to GMP manufacturing and global patient supply. If you require support with the development and manufacturing of an ATMP, then contact us today.
Author: Paul Crozier – January 2026
References:
www.alliancerm.org/resources/?_resource_type=cell-gene-therapy-sector-data
https://alliancerm.org/wp-content/uploads/2026/01/SOTI-2026-Industry-Update.pdf
www.citeline.com/en/resources/q3-2025-gene-cell-and-rna-therapy-report
https://www.eesc.europa.eu/en/our-work/opinions-information-reports/opinions/biotech-act
https://www.businessnewsaustralia.com/articles/mesoblast-shares-hit-five-year-high-as-ryoncil-quarterly-sales-surge-60-per-cent-to-52m.html
https://ashpublications.org/blood/article-abstract/146/16/1897/546856/Remestemcel-L-rknd-Ryoncil-the-first-approved?redirectedFrom=fulltext

by Paul Crozier | Jan 14, | HiTech Health Latest News
In the European Union (EU), the release of medicinal products for commercial supply is a highly regulated activity. Central to this release process is the Qualified Person (QP), a legally defined role under EU pharmaceutical legislation. The QP is responsible for ensuring that every batch supplied to the market meets the required standards of quality, safety, and compliance.
What Is a Qualified Person?
A Qualified Person is an individual named on a Manufacturer’s / Importer’s Authorisation (MIA) who meets specific education, training, and experience requirements to fulfil the role. The QP acts as the final accountable authority for batch certification prior to commercial distribution within the EU.
Key Responsibilities in Commercial Batch Release
Prior to certifying batches for market supply, the QP has to ensure:
- The medicinal product has been manufactured and tested in accordance with EU GMP and the approved Marketing Authorisation (MA).
- All manufacturing, testing, and packaging steps have been completed and documented in line with GMP requirements.
- Any deviations, changes, or out-of-specification results have been appropriately investigated and resolved.
- For imported products, manufacturing and quality controls conducted outside the EU are equivalent to EU GMP requirements.
Why the QP Role Matters?
The QP function is a critical component of the EU pharmaceutical regulatory system. It provides regulators and patients with assurance that medicinal products released to the market consistently meet EU standards, regardless of where they are manufactured. The role of the QP carries legal responsibility, with individual accountability to EU competent authorities for each certified batch. This responsibility underpins the assurance that product quality and patient safety consistently meet the highest regulatory standards.
For companies supplying medicinal products in the EU, access to an experienced QP team will help ensure timely and uninterrupted commercial supply. HiTech Health is a leading provider of QP services in the EU and also the UK. Our QP team have extensive knowledge of pharmaceuticals, biologics and Advanced Therapeutic Medicinal Products (ATMPs) including cell and gene therapies. We can help with all quality and compliance activities from your overall quality management system through quality audits and QMS remediation support.
Contact our team today and schedule a call to learn more about our QP services for the EU and UK markets.

by Paul Crozier | Dec 11, | HiTech Health Latest News

Over the past decade, the regenerative medicine landscape has undergone an extraordinary evolution with the continuous growth of Advanced Therapeutic Medicinal Products (ATMPs). ATMPs are medicines for human use that are based on genes, tissues or cells. They treat the root cause of diseases and disorders by altering, augmenting, repairing, replacing, or regenerating organs, tissues, cells, genes and metabolic processes in the body. This means that they can offer potentially groundbreaking new opportunities to address unmet medical needs.
10 years ago, in 2014, the ATMP sector was starting to grow but several hurdles including development, manufacturing and regulatory challenges and uncertainties needed to be addressed. Back then the field was still in its infancy, with only a few approved therapies and a rapidly growing number of early stage development programmes. The FDA had approved a small number of cell therapies, including several cord blood-based products like Hemacord and Allocord. However, there were no FDA-approved gene therapies in 2014.1
So much has changed in a positive way for patients. In 2024, the ATMP sector has expanded significantly, with therapies now available for a diverse range of medical conditions and they have become more accessible. There have been major improvements in the development, manufacturing and supply chains of ATMPs that have helped more patients receive these lifesaving and life changing medicines. There are now multiple gene therapies on the market for rare genetic disorders, such as Spinal Muscular Atrophy and Duchenne Muscular Dystrophy.
The sector has made significant advancements in 2024, driven by positive regulatory approvals, breakthroughs in scientific research, manufacturing optimisations and progress in market access and pricing. ATMPs have already saved and improved many lives, for example, a treatment for spinal muscular atrophy, Zolgensma, has been delivered to over 3,700 children globally. The sector has shown robustness and promising foundations for continued sector growth in 2025 and beyond. Developers are not only focused on proving that therapies can work but, are placing increased emphasis on refining their effectiveness, reducing side effects, expanding their indications and addressing market access and cost challenges.2
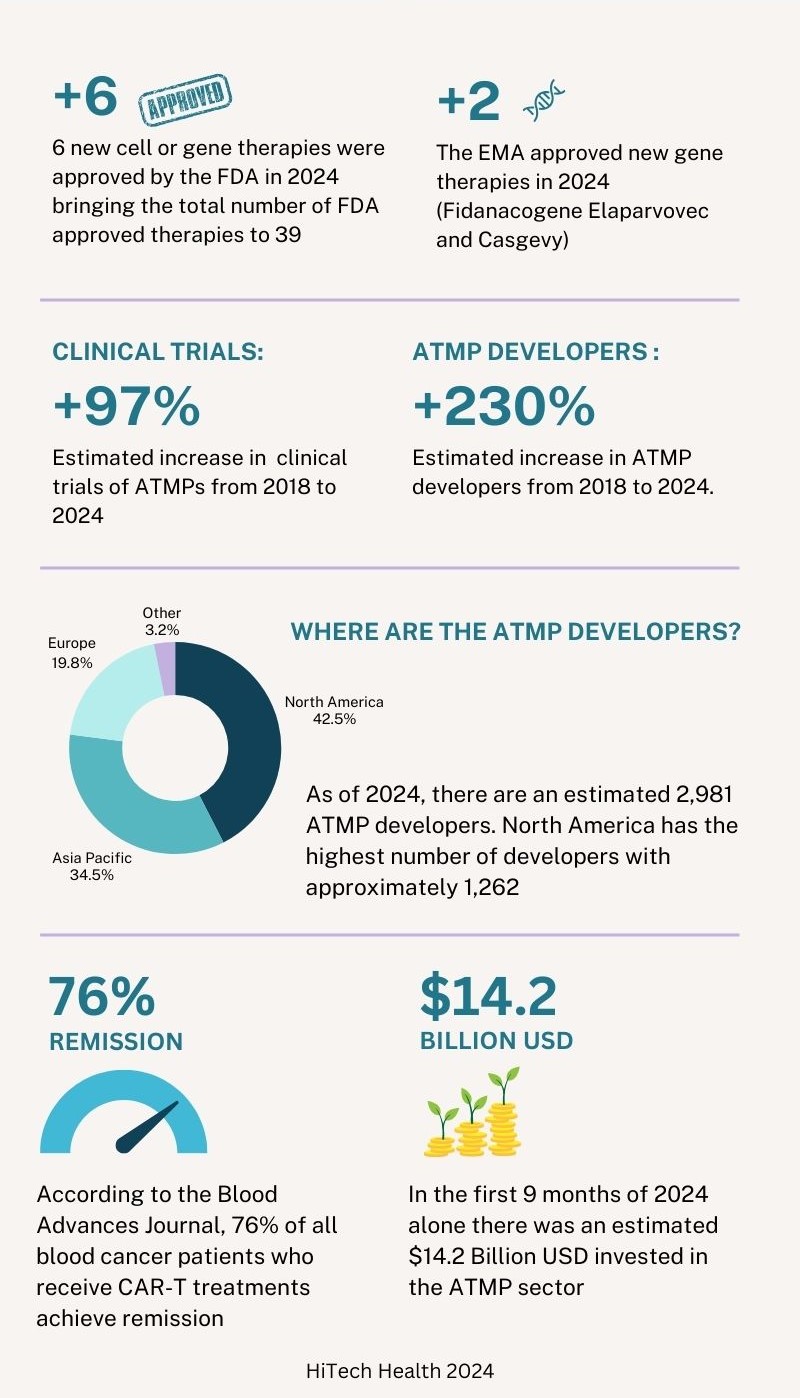
At the end of 2024, there were 39 cell and gene therapies approved in the United States by the FDA. Of the 6 new therapies approved by the FDA in 2024, 3 were gene therapies and 3 were cell therapies. In 2024 there have also been 2 new gene therapies approved by the European Medicines Agency (EMA). In addition to this, the FDA, EMA and other regulatory agencies are actively reviewing submissions made in 2024 and it is expected that a number of approval decisions will be made in the first quarter of 2025. The table below shows the therapies that were approved by the FDA and EMA in 2024.3
| Therapy |
Type |
Indication |
Developer |
Approval Status |
| Lifileucel |
Cell Therapy |
Metastatic Melanoma |
Iovance Biotherapeutics |
FDA approved 16 February 2024 |
| Lenmeldy |
Gene Therapy |
Metachromatic Leukodystrophy |
Orchard Therapeutics |
FDA approved 18 March 2024 |
| Fidanacogene Elaparvovec |
Gene Therapy |
Hemophilia B |
Pfizer |
FDA approved 27 April 2024
EMA approved 25 July 2024 |
| Tecelra |
Cell Therapy |
Advanced Synovial Sarcoma |
Adaptimmune Therapeutics |
FDA approved 1 August 2024 |
| Obecabtagene Autoleucel |
Cell Therapy |
B-Cell Acute Lymphoblastic Leukaemia |
Autolus Therapeutics |
FDA approved 8 November 2024 |
| Kebilidi |
Gene Therapy |
Aromatic L-Amino Acid Decarboxylase Deficiency |
PTC Therapeutics |
FDA approved 14 November 2024 |
| Casgevy |
Gene Therapy |
Sickle cell Disease and Beta-Thalassemia |
Vertex Pharmaceuticals and Crispr Therapeutics |
EMA approved 13 February 2024
(Previously approved by the FDA in late 2023 with an expanded label approval on 16 January 2024) |
Six years ago, in 2018, there were approximately 1000 clinical trials and 900 developers across the ATMP sector. By the end of the third quarter of 2024, the number of clinical trials has increased by approximately 97% in 6 years with an estimated 1,968 clinical trials. The number of developers has increased by approximately 230% during the same period to 2,981 developers. Of this estimated number of developers, 1,262 are based in North America, 1,036 are based in Asia Pacific, 587 are based in Europe and 96 are in other regions. Compared to the data in 2023, ‘other regions’ outside North America, Asia Pacific and Europe have seen the largest growth with an estimated yearly increase of 12.9% followed by Asia Pacific (12%), North America (6.6%) and Europe (3.3%).3
Similar to 2023, the majority of the clinical trials still involve cell-based therapies, accounting for approximately 70% of all trials. Oncology remains to be the most prevalent indication for ATMPs. Cancer patients, especially those who relapse, often must endure a series of arduous treatments using current standard of care chemotherapy and radiotherapy. CAR-T therapies have shown a reduction in collateral damage to healthy cells and can minimise side effects compared to traditional treatments. According to the Blood Advances Journal, 76% of all blood cancer patients who receive CAR-T treatments achieve remission. For B cell Lymphoma, overall survival is almost 9 times higher than the standard of care. Patients who receive Yescarta, a therapy used to treat B cell lymphoma, are 21% less likely to require subsequent treatment (insert reference: Vanderbilt University).3 4
Looking into the investments made in the ATMP sector, there was an estimated $13.3 billion USD invested 6 years ago in 2018 (excluding mergers and acquisitions (M&A) and grants). In the first 9 months of 2024, there has been a total estimated investment of $14.2 billion. At the time of writing, the data for the final 3 months of 2024 is not yet available but it is projected that the total annual investment will surpass $17 billion USD. There have been fluctuations in total investment over the last several years but the sector has shown robustness and positive investor sentiment. Across all regions, the largest type of investment is in the form of venture financing. North America has consistently seen the highest levels of investment since the industry was first developed.3
In Europe, in January 2022, Regulation (EU) 2021/2282 on health technology assessment (HTA) came into effect. The regulation aims to be applied in EU Member States by next month on the 12th January 2025. The HTA introduces an evidence‑based process that will allow the competent EU and national authorities to determine the effectiveness of new or existing health technologies, including cell and gene therapies. From 2025, all cell and gene therapies will undergo a single EU assessment of the value they add to patients and healthcare systems, aiming to end the need for 27 individual reviews. Companies will also meet jointly with the European Medicines Agency and Europe’s HTA coordinating group to discuss and align on the optimal clinical trial designs that deliver data, not only on safety and efficacy but also on added value to patients and healthcare systems.5
The regulation of ATMPs has progressed significantly in both the US, Europe and Asia Pacific regions over the last several years. In the US, the FDA has defined more transparent pathways for ATMPs, supported by the Regenerative Medicine Advanced Therapy designation, which facilitates expedited review and development support. The EMA has refined its guidelines for ATMPs, publishing a joint action plan with the European Commission to offer more streamlined processes for developers. There are opportunities for improvements in the regulatory pathways and we expect that the coming years will yield further changes. Developers are seeking more harmonised international regulations to help them navigate approvals across multiple regions more effectively. Notably, the regulatory agencies have also responded to the growing need for post-market surveillance, recognising the long-term nature of many ATMPs and the requirement for ongoing monitoring of their safety and efficacy.
2024 continues the trend of significant advances in the development and approval of new ATMPs which, ultimately, is great news for patients. The number of therapies receiving regulatory approval remains high with several applications still under review by the agencies. While there are challenges for ATMPs in manufacturing, pricing, and safety, the significant increase in the number of clinical trials demonstrates the potential of these medicines to enhance and transform patients’ lives. The outlook for the sector remains strong, with continued investment, technological innovation, and regulatory support propelling the industry forward in 2025 and beyond.
Hitech Health is a leading European CDMO and service provider for ATMPs. If you require support with the development and manufacturing of an ATMP, schedule a meeting with our team by emailing info@hitech-health.com.
References:
1 – https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
2 – https://www.novartis.com/news/media-releases/novartis-presents-new-data-safety-and-efficacy-zolgensma-including-maintained-and-improved-motor-milestones-older-and-heavier-children-sma
3 – https://alliancerm.org/data/
4 – https://www.hematology.org/newsroom/press-releases/2023/a-promising-outlook-car-t-cells-improve-patient-quality-of-life
5 – https://health.ec.europa.eu/health-technology-assessment/implementation-regulation-health-technology-assessment/joint-clinical-assessments_en

by Paul Crozier | Nov 19, | HiTech Health Latest News
IDA Ireland, the agency responsible for the attraction and retention of inward foreign direct investment (FDI) into Ireland, has recently published an article entitled ‘Why Ireland is a proving ground for medicinal breakthroughs like cell and gene therapy’. Hitech Health is delighted to be featured as part of Ireland’s exciting growth in the cell and gene therapy space. Hitech was the first commercial company to have a licence to manufacture these advanced medicines in Ireland. It works with some large global companies, but Managing Director Brian Harrison says most of the companies in the cell and gene therapy space are smaller companies including university spin outs.
In many cases, these advanced medicine companies have products in development, and need expertise to manufacture pre-clinical materials as is required for toxicology studies. Hitech Health can also manufacture clinical trial materials for these advanced medicine companies once the potential treatment has been approved by regulators to be used in patients . In some cases, large pharma companies outsource these advanced medicine products to contractors and they continue to use Hitech for support.
Read the full article here:https://www.idaireland.com/latest-news/insights/why-ireland-is-a-proving-ground-for-medicinal-breakthroughs

by Paul Crozier | Jul 28, | HiTech Health Latest News
Many Cell and Gene Therapy (CGT) companies outsource product development and manufacturing activities to a Contract Development and Manufacturing Organisation (CDMO). It is critical to ensure that an informed decision is made when selecting a CDMO to prevent future disruption to the intricate supply chains of cell and gene therapies and ultimately patients. HiTech Health explores 5 key considerations for a cross-functional team to examine when partaking in the CDMO selection process.
- Does your CDMO have an experienced CGT team?
The complex and time-sensitive nature of CGT manufacturing processes makes it imperative to select a CDMO with a team who have deep knowledge of cell and gene therapies. The technical and regulatory landscapes in the CGT industry are changing frequently and ensuring your CDMO has in-depth expertise and flexibility is crucial to success. Assessing the CGT experience of the proposed CDMO team could reduce the risk of knowledge gaps later. A CDMO may have a strong track record of developing and manufacturing small molecule products and biologics but may have limited experience of CGTs. Are sufficient resources in place now and into the future?
- Is there an integrated project management approach defined by the CDMO?
If you have shortlisted potential CDMO partners, have you enquired about their project management approach? How do the proposed approaches differ among the potential partners? Having an integrated project management approach that ensures seamless alignment between production, supply chain, QC testing personnel and many other functions is paramount given the stringent timeframe for supplying CGTs to patients. Understanding how frequently you will meet with the assigned CDMO project manager and how they will communicate with you should be clearly defined from the beginning.
- Does the CDMO practice effective Quality by Design (QbD)?
Considering if a CDMO practices Quality by Design (QbD) is an important part of the due diligence process. The CDMO team may work closely with you to measure, analyse and report on the quality attributes of the CGT product. The team members who develop your upstream regulatory strategy and initial CMC plan need to be able to work closely with a trusted CDMO partner who can ensure quality and compliance goals are met. Have you considered an on-site audit of the CDMO before the final selection?
- How flexible is the potential CDMO partner?
During the due diligence assessment, look at the CDMO’s internal systems and workflows for a true indication of their fit as a potential partner. The CDMO should be experienced and agile to help you navigate unforeseen challenges, open and transparent to help you mitigate risks and control your costs as well as being fully committed to the ultimate goal of providing CGTs to the patients who vitally need them. These are the characteristics of a resilient and long-lasting partnership, and the indicators of future success.
From the start of a project, the right CDMO will encourage an ongoing dialogue about the quality, pace and cost considerations of your project. They will focus on developing a robust and scalable approach that will work for the entire lifecycle of your project.
- Is there a Business Continuity Plan (BCP) that has been implemented and managed effectively by the CDMO?
The CDMO’s ability to maintain essential functions during and after an unforeseen event has occurred is very important and should be assessed prior to signing a contract. Business continuity planning, in this case, relates to establishing risk management processes and procedures that aim to prevent interruptions to manufacturing and supplying CGTs. If an unforeseen event occurs, such as critical components not being available, how will the CDMO mitigate or manage the issues to ensure the effects on operations are minimised. Have you requested a copy of your shortlisted CDMO’s business continuity plan?
Learn more about our cell and gene therapy services by clicking here
Find us on LinkedIn
Contact: info@hitech-health.com