
by Yanan Zhu | Feb 14, | HiTech Health Latest News
Ireland, 12th Feb 2024: Hitech Health, a Contract Development and Manufacturing Organisation (CDMO) for advanced therapies, has opened an additional facility in County Galway, Ireland. The new high-specification manufacturing centre is equipped with state-of-the-art technologies to help develop, manufacture and test sterile formulations including cell and gene therapies. Cell and gene therapies offer revolutionary treatments which repair, replace, regenerate and re-engineer genes, cells and tissues to restore normal function or enhance their ability to fight diseases, like cancer.
The new manufacturing centre operates to Good Manufacturing Practice (GMP) standards and is certified to European Medicine Agency (EMA) standards by the Health Products Regulatory Authority (HPRA). It will provide the global Advanced Therapies industry with GMP manufacturing services for their clinical studies, helping to accelerate these novel therapies through commercialisation. The multi-disciplinary team at Hitech Health have extensive experience helping Advanced Therapy developers progress their therapies to help treat unmet medical needs.
The new manufacturing centre adds to Hitech Health’s capabilities for our clients that have been supported since the company was founded in 2013. Hitech Health’s expert team are working with clients including in the US, Europe and the United Kingdom. We are responsible for the certification, release and supply of multiple different cell and gene therapies that are currently in clinical trials.
Brian Harrison, Managing Director of Hitech Health, commented:
“I am delighted to announce the opening of this manufacturing centre that offers our clients capacity for GMP manufacture of these life changing medicines for treating patients. Partnerships and collaboration are at the core of successful research and innovation and this is a major focus for Hitech Health. Advanced Medicines including cell and Gene Therapy products is the fastest growing sector of new product development. There were 7 new advanced medicines approved by the FDA in 2023. This number is expected to increase to between 10-20 per year by 2025. Hitech Health is a company with the capability to support the development of these new therapies in this fast growing area of medicines which is pivotal for treating patients with rare disease.
Learn more about our services by clicking here
Find us on LinkedIn
Schedule a meeting by emailing : info@hitech-health.com

by Yanan Zhu | Dec 13, | HiTech Health Latest News
The Advanced Therapeutic Medicinal Products (ATMPs) sector is evolving rapidly and new products have already demonstrated the ability to reverse or significantly impact the progression of disease. In recent years, the advent of cell and gene therapies has shown the possibility of providing transformative and potentially curative outcomes for a diverse range of diseases and injuries.
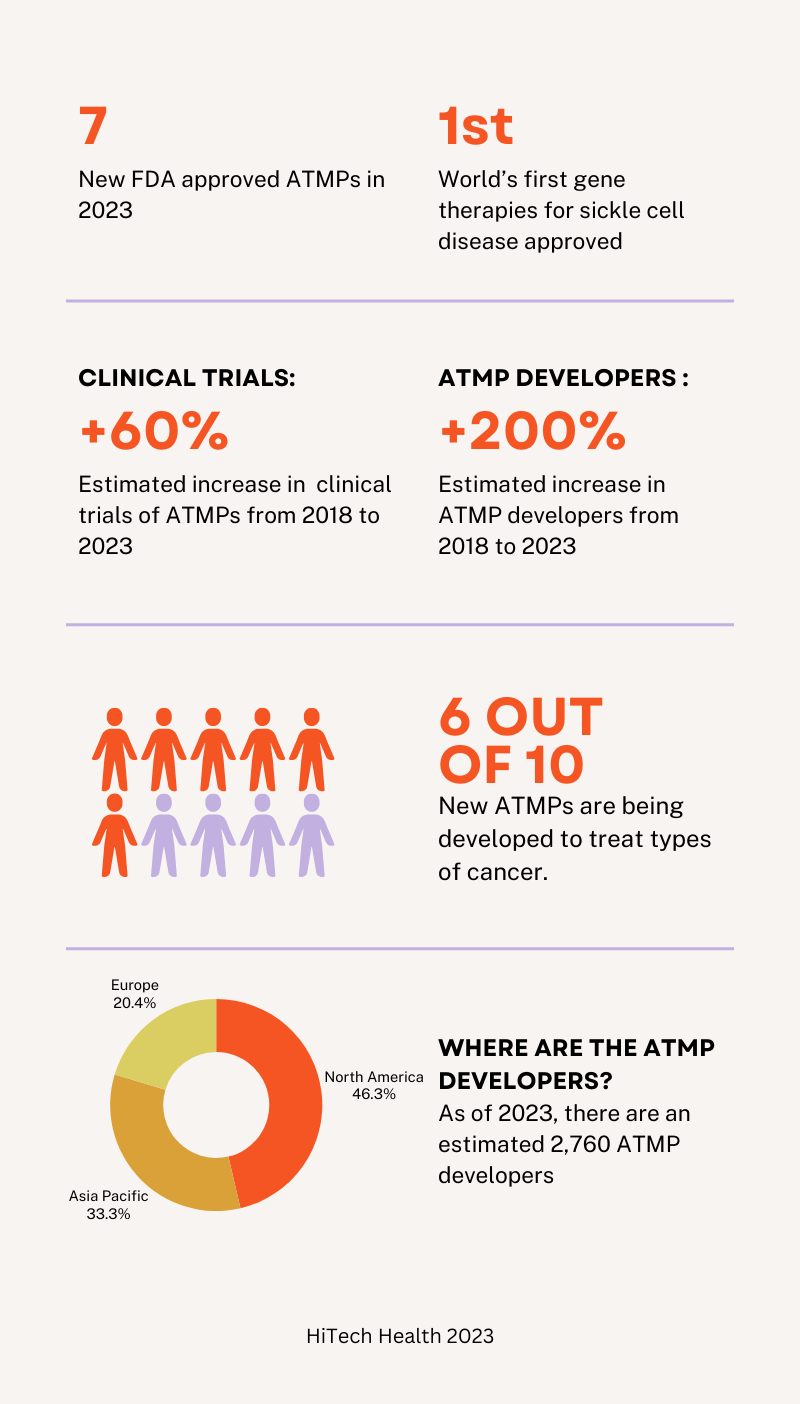
At the end of 2022, there were 26 cell and gene therapies approved in the United States by the FDA. In 2023, an additional 7 cell and gene therapies were approved bringing the total number to 33 approved therapies (1). The largest sub-class of approved therapies are umbilical cord blood derivatives, representing 8 of the 32 cell and gene therapy approvals to date. CAR-T cell therapies represent the next largest segment, composing 6 of the 32 cell and gene therapy approvals (2). The approved CAR-T cell therapies in the U.S. include: Abcema, Breyanzi, Carvykti, Kymriah, Tecartus, and Yescarta.
In December 2023, the FDA approved the first gene therapies, Casgevy and Lyfgenia, for sickle cell disease in patients 12 years or older. Casgevy was developed through a partnership with Vertex Pharmaceuticals and CRISPR Therapeutics while Lyfgenia was developed by Bluebird Bio. Both therapies are made from the patients’ own blood stem cells, which are modified, and are given back as a one-time, single-dose infusion as part of a hematopoietic (blood) stem cell transplant. Prior to treatment, a patients’ own stem cells are collected, and then the patient must undergo myeloablative conditioning (high-dose chemotherapy), a process that removes cells from the bone marrow so they can be replaced with the modified cells in Casgevy and Lyfgenia (3). Casgevy was already approved by the UK’s MHRA on 15th November 2023.
The 33 cell and gene therapies that have been approved by the FDA represent approximately 9% of the estimated 359 approved biologics (4). Although this is a small percentage it does mark significant growth considering the first FDA approval of a cell and gene therapy occurred in 2017, Kymriah.
The FDA has identified a requirement for additional qualified people to manage the growing number of cell and gene therapy submissions. There are currently more than 1,000 cell and gene therapies in clinical development in the U.S., with more than 3,000 in pre-clinical development (5). To meet the growing demands, the US congress authorised new funding for the agency when it reauthorised the new Prescription Drug User Fee Act (PUFA VII) in 2022. This has allowed the FDA to pursue the hiring of new employees for its newly created ‘super office’, named the Office of Therapeutic Products, which replaced the Office of Tissues and Advanced Therapies.
New offices created within the super office structure align disciplines and product types, aiming to allow the FDA’s workforce to address the exponential growth in cell and gene therapies. The transition to the new Office of Therapeutic Products aims to help the FDA meet its expectation of approving 10-20 cell and gene therapies annually by 2025.
In Europe, the EMA had approved 24 ATMPs by the end of 2022, although 7 of the products were subsequently withdrawn from the market or else did not have their Marketing Authorisation (MA) renewed (6). The first ATMP to be approved by the EMA in 2023 was Hemgenix. Hemgenix was developed by Dutch biotechnology company UniQure to treat adults with severe and moderately severe haemophilia B, an inherited bleeding disorder caused by the lack of factor IX (a protein needed to produce blood clots to stop bleeding) (7).
In January 2022, Regulation (EU) 2021/2282 on health technology assessment (HTA) came into effect. The regulation aims to be applied by 12th January 2025 and will therefore take three years to become effective in Member States. The HTA introduces an evidence�based process that will allow the competent EU and national authorities to determine the effectiveness of new or existing health technologies, including cell and gene therapies. From 2025, all cell and gene therapies will undergo a single EU assessment of the value they add to patients and healthcare systems, aiming to end the need for 27 individual reviews. Companies will also meet jointly with the European Medicines Agency and Europe’s HTA coordinating group to discuss and align on the optimal clinical trial designs that deliver data, not only on safety and efficacy but also on added value to the patients and healthcare systems (8, 9).
In 2018, there were approximately 1000 clinical trials and 900 developers according to the Alliance for Regenerative Medicine. As of 2023, there are an estimated 1,687 clinical trials that are on-going and an estimated 2,760 developers. Of the 2760 developers, 1,235 are based in North America, 888 are based in Asia Pacific and 543 are based in Europe. Of the current active clinical trials, 917 trials are in North America, 648 are in the Asia Pacific region and 329 are in Europe (10).
The majority of the clinical trials involve cell therapies or gene-modified cell therapies, accounting for approximately two thirds of the trials. The rest of the trials involve DNA and RNA therapeutics, gene therapies and genone editing. Types of cancer are the most common therapeutic indication, approximately 60% (10).
The data from 2023 shows clear growth in the ATMP sector that is yielding positive outcomes for patients. Over the last several years, the ATMP sector has seen consistently increasing numbers of clinical trials, increases in investment, partnerships, and year-on-year growth in regulatory approvals by both the FDA and the EMA. Challenges undoubtedly lie ahead in 2024 but it is clear that ATMPs are helping patients with unmet medical needs. The strong pipeline of future therapies requires continuous innovation and investment by the regulatory agencies, developers and manufacturers to meet the industry’s requirements.
Hitech Health is a leading European CDMO and service provider for ATMPs including cell and gene therapies. If you require support with the development and manufacturing of an ATMP, schedule a meeting with our team by emailing info@hitech-health.com.
Author: Paul Crozier
Date: 13th December 2023
References
- fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
- bioinformant.com/u-s-fda-approved-cell-and-gene-therapies
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
- cellandgene.com/doc/s-market-outlook-for-cell-and-gene-therapies-0001
- biospace.com/article/fda-braces-for-looming-boom-in-cell-and-gene-therapy-submissions
- ema.europa.eu/en/documents/report/cat-quarterly-highlights-approved-atmps-may-2023_en.pdf
- ema.europa.eu/en/medicines/human/EPAR/hemgenix
- https://www.europeanpharmaceuticalreview.com/article/173762/the-eu-hta-regulation-a-new-frontier-for-access-to-innovative-technologies/
- politico.eu/article/europe-afford-next-generation-medicine/#:~:text=me%20the%20money-,To%20date%2C%20the%20European%20Commission%20has%20authorized%2025%20cell%20and,and%20certain%20types%20of%20cancer.
- alliancerm.org/the-sector-snapshot-august-2023/

by Yanan Zhu | Dec 7, | HiTech Health Latest News
HiTech Health is thrilled to announce its partnership with VascVersa, a trailblazer in regenerative medicine focusing on pioneering cell therapies that significantly enhance vascular regeneration and repair. Originating from Queen’s University Belfast, VascVersa is a dynamic spin-out backed by over two decades of meticulous research. This extensive study has culminated in the development of innovative cell therapies that stand at the forefront of medical science. Their flagship product, ANGICYTE™ Technology, is a revolutionary cell therapy approach aimed at blood vessel regeneration. This advanced treatment is designed to stimulate the formation of new blood vessels, which in turn improves blood supply to affected areas and facilitates long-term healing and recovery.
This cutting-edge cell therapy boasts a wide range of potential applications, promising transformative impacts across various ischaemic diseases. The ANGICYTE™ Technology harnesses the power of targeted cell therapy to address the root causes of vascular deficiencies, thereby enhancing patient outcomes and quality of life. The HiTech Health team is enthusiastic about the prospects of this collaboration with VascVersa. Together, they aim to push the boundaries of what’s possible in cell therapy, advancing these transformative treatments through clinical trials to real-world application. By utilizing ANGICYTE™ Technology, HiTech Health is committed to developing effective treatments that offer hope and healing to patients suffering from vascular issues.
Learn more about our cell and gene therapy services by clicking here

by Yanan Zhu | Nov 24, | HiTech Health Latest News
The Disruptive Technologies Innovation Fund (DTIF) is a €500 million fund established under Ireland’s National Development Plan (NDP) in 2018. On Thursday 23rd November, Ireland’s Minister for Enterprise, Trade and Employment, Simon Coveney TD, Minister for Further and Higher Education, Research, Innovation and Science, Simon Harris TD and the Minister of State for Trade Promotion, Digital and Company Regulation, Dara Calleary TD, today announced funding of a further €58.8 million to 12 new projects under the DTIF.
The purpose of the fund is to drive collaboration between Ireland’s world-class research base and industry as well as facilitating enterprises to compete directly for funding in support of the development and adoption of these technologies. The aim is to support investment in the development and deployment of disruptive technologies and applications on a commercial basis.
HiTech Health (Déantúsaíocht Sláinte Hitech Teoranta), University of Galway (UG) and Odyssey Validation Consultants Ltd have been awarded €6,579,772 as part of the DTIF for a CellConnect project. CellConnect will capture data from every stage of the cell manufacturing process and empower Ireland’s cell-based therapeutic manufacturing revolution by developing disruptive methods to automate processes and transport cell products.
Dr Brian Harrison, Managing Director of HiTech Health, said “We are delighted to have been given this prestigious DTIF award and look forward to working with our partners, the Duffy Laboratory in University of Galway and Odyssey Validation Consultants to bring this exciting CellConnect project to reality. Cell Therapies really are life changing medicines and Hitech Health are currently working with several client companies who are bringing new therapies to patients many of whom suffer from rare diseases. This CellConnect platform will provide a significant step forward in the automation of processes, many of which are currently paper based. This project will also build capability in Ireland in this rapidly growing area of ‘Advanced’ medicines which is leading to treatments that can transform patients’ lives”.
Read the full press release here
Find us on LinkedIn
Contact: info@hitech-health.com

by Yanan Zhu | Nov 14, | HiTech Health Latest News
Hitech Health is delighted to be attending Advanced Therapies Integrates in Stevenage, England on the 30th November. This event aims to drive the translation of cutting-edge research and disruptive innovation into the wide-spread adoption of Advanced Therapies, delivering improved patient outcomes and a revolution in healthcare economics. Advanced Therapies Integrates will bring together innovators, regulators, supply chain partners, equipment & technology providers, academia, industry associations, together with a wide array of government funding bodies and government supported pharma-focused initiatives.
The genomics revolution and other advances have led to the emergence of highly effective, but extremely complex therapeutics, with cell and gene therapies, regenerative medicines and tissue therapies now transforming the prospects of patients and healthcare providers around the globe. As a full service CDMO for advanced medicines, Hitech Health is well positioned to work closely with you to support the successful development and manufacturing of new therapies.
To learn more about our cell and gene therapy services by clicking here
Find us on LinkedIn
Contact: info@hitech-health.com

by Yanan Zhu | Nov 9, | HiTech Health Latest News
Afortiori Development and HiTech Health Forge an Important Collaboration to Support the Clinical Research and Product Development of Groundbreaking Cell and Gene Therapies.
Galway, November 9, 2023 – Afortiori Development, a premier full-service Clinical Research Organisation, is thrilled to announce its latest partnership with HiTech Health, a leading Contract Development and Manufacturing Organisation (CDMO) for Advanced Therapy Medicinal Products (ATMP) including Cell and Gene Therapies (CGT) (Europe/UK/Ireland/US). This strategic alliance aims to provide an integrated suite of services to companies conducting research in the ATMP and CGT areas. The two companies believe that by seamlessly integrating clinical trial expertise with cutting-edge product development and manufacturing capabilities, this will provide the calibre of service required by companies conducting research, ensuring that their clinical research journey is enhanced overall.
Afortiori Development’s unwavering commitment to providing expert, flexible and economically viable clinical trial design and clinical trial management services perfectly complements HiTech Health’s extensive knowledge and expertise in product development, manufacturing and supply chain. By joining forces, these industry pioneers are in a strong position to deliver unparalleled support, enabling clients to optimise their clinical research processes, enable faster trial start up and drive ongoing support of their revolutionary products while research is in progress.
“Our approach has to evolve to deliver the cutting-edge services that clients innovating in the advanced therapy space require. Aligning our services with companies like HiTech Health means that we can ensure timely and high-quality supply of products required for trial needs. A critical requirement for many of these advanced cell and gene therapies,” emphasised Dr Nicola Wall, CEO at Afortiori Development. “We understand the paramount importance of obtaining high-quality IMP that is available when needed for researchers and meets the quality standards. Through our collaboration with HiTech Health, we are poised to provide unmatched expertise and support to our clients running these types of clinical trials.”
Afortiori Development and HiTech Health represent a combined team of seasoned experts who work closely with clients, offering flexible, scalable, and tailored solutions to each trial’s specific needs. Throughout the entire process, these industry leaders remain dedicated advocates for their clients, ensuring they benefit from the optimal clinical trial delivery services that generate actionable insights for safety, efficacy and regulatory compliance.
“The success of the clinical trial is deeply affected by the timely availability of high quality products, supported by the right processes, systems, and experts,” added Dr Brian Harrison, CEO of HiTech Health. “Through our collaboration with Afortiori Development, clients can leverage the combined strength of a team that knows exactly what needs to happen and when, ensuring our projects meet timelines and expectations overall.”
Both companies encourage clients to explore the combined team’s extensive knowledge and capabilities in trial design, trial delivery operational planning, product development and Chemistry Manufacturing and Controls (CMC) operational activities. With HiTech Health’s state-of-the-art laboratories and GMP manufacturing facilities equipped for process and analytical development, clients can rely on their cutting-edge cell and gene therapy expertise to accelerate product development timelines.
Afortiori Development and HiTech Health invite clients and industry professionals to get in touch to discuss their current and future needs. Engaging with their dedicated teams of experts will unlock new possibilities and propel clients towards success on this exhilarating path.
About Afortiori Development:
Afortiori Development is a full-service Clinical Research Organisation specializing in the design and management of clinical trials and post-market clinical follow-up studies. With a flexible, scalable, and client-centric approach, Afortiori Development provides critical support to clients to facilitate the right clinical trial.
About HiTech Health:
HiTech Health is a CDMO focused on product development and GMP manufacturing of advanced medicines including cell and gene therapy products.. Their specialized knowledge and state-of-the-art facilities enable efficient and cost-effective development and manufacturing solutions for clients.
To learn more about our cell and gene therapy services by clicking here
Find us on LinkedIn
Contact: info@hitech-health.com







